Technology development.
(1). Metabolic imaging for cancer biology research: Being able to quantify tumor bioenergetics, in a live tumor microenvironment and being able to look at the evolution of the disease in the face of transient stress, is an important new direction and the new capabilities that this technology is poised to offer will have the potential to address these important questions in the cancer biology field. Our group has developed a multi-parametric microscope imaging toolbox that is able to quantify glucose uptake (2-NBDG fluorescence), mitochondrial membrane potential (TMRE fluorescence), and vascular structure and oxygenation in vivo in tumor models. This toolbox allows for monitoring of the similar metabolic axes as in vitro cellular metabolism analyzers, but with the benefit of repeated, nondestructive imaging within an intact microenvironment and at a length-scale and resolution that complements PET and MRI imaging. By imaging multiple key endpoints in vivo, our technique provides a more holistic view of metabolism than most metabolic imaging techniques that measure a single endpoint or lack resolution to view spatial relationships. Ultimately, our toolbox can be used to better understand metabolic reprogramming for cancer therapy.
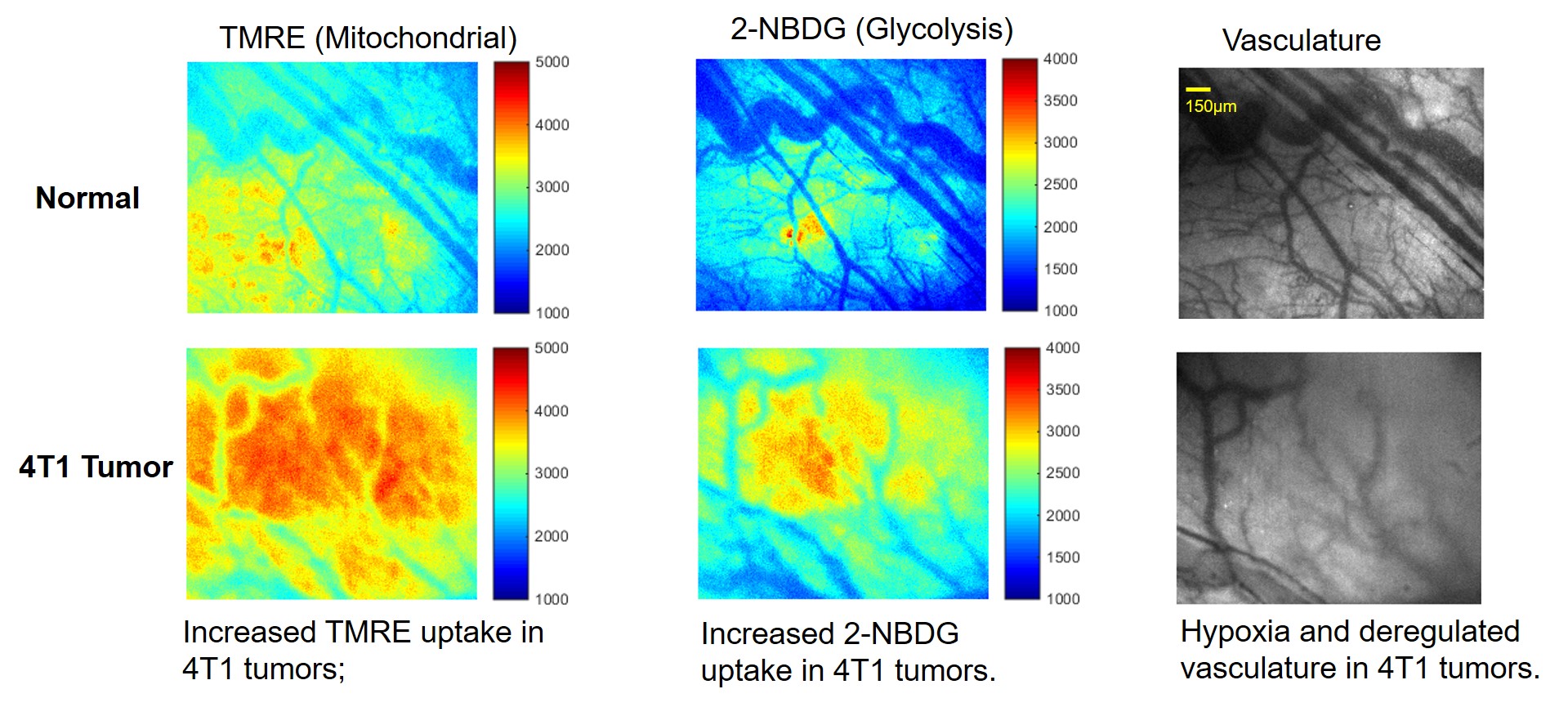
Novel strategy enables simultaneous imaging of glucose uptake and mitochondrial metabolism along with vasculature in vivo .
Founding support: NIH-COBRE, NCI-CCSG, ACS-IRG
(2). Optical spectroscopy for pharmacology research: Optical spectroscopy can leverage endogenous contrast or be coupled with appropriate indicators to provide quantitative endpoints related to tumor metabolism and its associated vasculature in vivo. Our optical technology is intended for pharmacology research in the cancer biology laboratory, the same setting as the Seahorse Assay, but with the added capability of being used for in vivo studies that are directly related to clinical tumors through PDX models. Our platform will enable novel cancer investigations, spanning fundamental inquiries to translational studies related to drug discovery and development as well as to assess the effectiveness of therapeutic interventions for clinical translation.
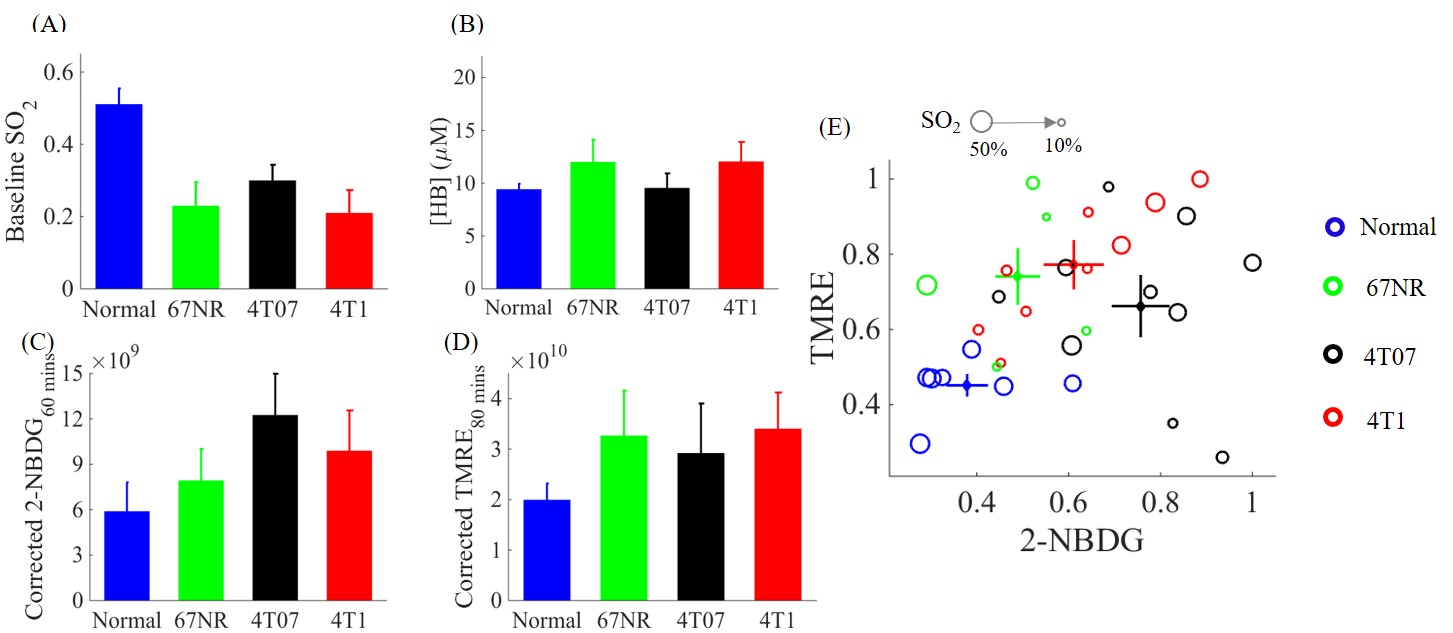
Simultaneous in vivo optical measure of key metabolic/vascular endpoints reveals tumor metabolic diversity in murine breast tumors.
Founding support: University of Kentucky startup, NIDCR R01
Cancer therapeutics.
(1). The role of metabolism reprogramming in radiation resistance for head and neck carcinomas: Radio-resistance (RR) leads to poor prognosis in head and neck squamous cell cancer (HNSCC) patients. Hypoxia-Inducible Factor-1a (HIF-1a) is known to regulate many growth factors to promote aerobic Radio-resistance (RR) leads to poor prognosis in head and neck squamous cell cancer (HNSCC) patients. We hypothesize that RT-induced HIF-1 expression and subsequent alterations in metabolism/vasculature underlie HNSCC RR. Unraveling metabolic traits of cells that evade RT and recur, and the role of the supporting vasculature, is critical to developing strategies to prevent HNSCC recurrence and improve patient survival. However, there are surprisingly few techniques available to provide a systems-level view of these hallmarks together in vivo. To fill these gaps, we will build a portable multi-parametric microscope to measure the major axes of metabolism and vasculature in small animal models in vivo. we will then use these platforms to study the effect of radiation on HNSCC tumors and test our hypothesis on HNSCC RR development. We envision that this system will be well suited to study tumor RR and recurrence in patient-derived xenograft (PDX) and organoid models, which can faithfully recapitulate many micro-environmental features of patient tumors.
Founding support: NIH-COBRE, NIDCR R01
(2). Early prediction of breast cancer radiation responses during BCS: Radiotherapy (RT) after BCS has emerged as an integral part of treatment for breast cancer (BC) patients, but the absolute benefit of RT is not equal for all patients. Moreover, RT increases the risk of secondary cancers such as sarcomas. Daily treatment adds to the costs, stress and time for patients, some of whom won’t benefit from the process. At present, all BC patients are treated with the same RT regimen, irrespective of whether their tumors are likely to respond or not. Currently, there is no way to tell which patients will benefit the most or the least before an actual RT, as no pre-evaluation criteria related to responsiveness exists. Increasing evidence shows that RT-induced metabolic changes might be responsible for RT resistance development. We propose the development of a more informed approach to evaluate individual responses to meet the urgent need for effective RT sensitivity pre-evaluation. In our state-of-art model, the primary tumor biopsy from the BCS will be cultured and assayed for RT sensitivity assessment through metabolic imaging on the cultured slices with RT stres. Several key metabolic parameters including glucose uptake, mitochondrial membrane potential (MMP), and bodipy uptake of tumor slices under RT stress will serve as effective biomarkers for RT sensitivity evaluation and potentially guiding treatment decisions and design. Our proposed approach would allow those patients likely to respond to receive higher doses of RT and/or radio-sensitizing agents to increase the efficacy of their treatment, while those patients unlikely to respond could be omitted from RT to minimize unnecessary side effects and reduce health expenditures with no therapeutic gain. The ultimate goal of our studies is to improve BC health outcomes and patient experience, and reduce health expenditures through providing personalized RT.
Founding support: University of Kentucky IRC 2020, NIBIB Trailblazer R21